Igdistributorspali@gmail.com - GST NO. : 08BUHPV5449L1ZR
- Send Email
Pharmaceutical Injection
Leading Wholesaler and Trader of Diclofenac Sodium Injection, Max One Single Use Syringe with Needle and Tetanus Vaccine from Pali.
| Business Type | Supplier, Trader |
| Form | Liquid |
| Purity | 99.99% |
| Country of Origin | India |
| Manufacturer By | Harson Laboratories |
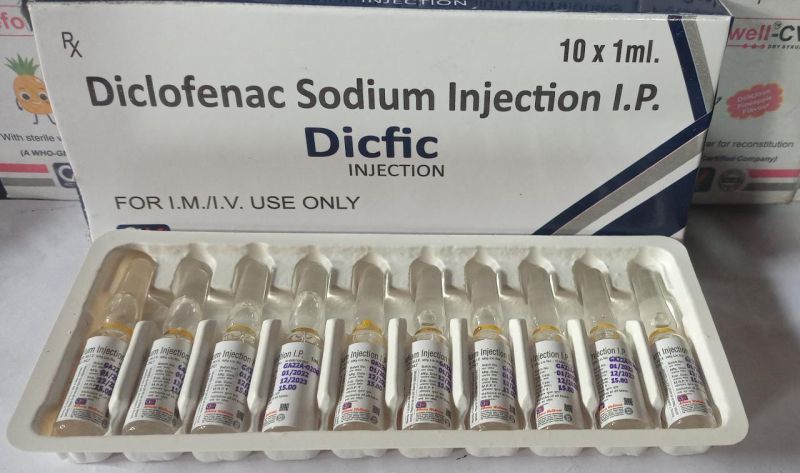
| Composition | Diclofenac |
| Generic Name | Dicfic |
| Packaging Type | Vial |
| Packaging Size | 10x1ml |
| Usage | Pain Relief |
| Strength | 75mg |
| Storage Requirements | Cool And Dry Place |
| Product Code | Diclofenac379 |
Diclofenac Sodium 75mg
| Business Type | Supplier, Trader |
| Brand Name | Max One |
| Country of Origin | India |
| Packaging Type | Paper Box |
| Feature | Certificate Of Surveillance, Good Quality, Rotating Connected |
| Needle material | Stainless Steel |
| Capacity | 2ml,3ml, 5ml, 10ml |
| Needle Gauge | 18G, 21G, 23G, 25G |
| Needle Length | 0.5 Inch, 1 Inch, 1.5 Inch |
| Expiration | 5 Years |
| Storage | Store In A Cool, Dry Place |
| Business Type | Supplier, Trader |
| Form | Liquid |
| Grade Standard | Medical Grade |
| Packaging Type | Vial |
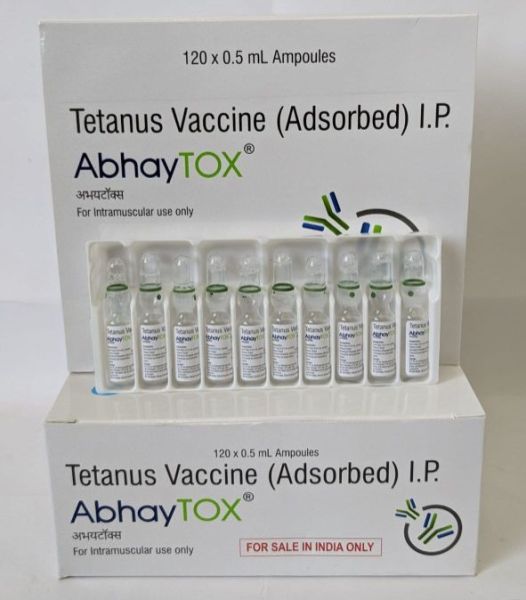
| Packaging Size | 120× 0.5ml Ampoules |
| Composition | Tetanus |
| Country of Origin | India |
| Manufacturer By | Indian Immunologicals Ltd. |
| Generic Name | AbhayTOX |
| Strength | 0.5ml per Ampoule |
| Side Effects | Pain, Redness, Swelling At Injection Site,Low-grade Fever,Irritability, Fatigue,Severe Allergic Reaction (anaphylaxis),Neurological Complications (extremely Rare) |
| Storage | +2°C To +8°C (refrigerated, Not Frozen),From Light,Keep Out Of Reach Of Children |
Composition (per 0.5 mL dose)
Tetanus Toxoid (adsorbed on aluminium phosphate/aluminium hydroxide gel): Equivalent to at least 40 IU
Aluminium phosphate / hydroxide (as adjuvant): Helps boost immune response
Preservatives (e.g., thiomersal or 2-phenoxyethanol depending on batch)
(Exact composition may vary slightly depending on manufacturer batch – but this is the standard for AbhayTOX.)
Indications (Uses)
Provides active immunization against tetanus.
Given for:
Routine childhood immunization schedule (as DTP/DTaP or standalone booster)
Wound management (to prevent tetanus in case of injury, cuts, or burns)
Booster doses every 10 years for continued protection
Dosage & Schedule
Primary immunization: Usually given as part of DTP vaccine in infants at 6, 10, and 14 weeks of age.
Booster doses:
1st booster at 16–24 months
2nd booster at 4–6 years
Further boosters every 10 years in adulthood
Wound prophylaxis: Single 0.5 mL dose if last vaccination was >5 years ago
(Exact schedule may vary as per Indian National Immunization Schedule and WHO guidelines.)
Side Effects
Mild (common):
Pain, redness, swelling at injection site
Low-grade fever
Irritability, fatigue
Rare (serious):
Severe allergic reaction (anaphylaxis)
Neurological complications (extremely rare)
Precautions & Warnings
Should be given only by a healthcare professional
Intramuscular injection – usually in anterolateral thigh (infants) or deltoid muscle (adults)
Avoid use in patients with:
Severe allergic reaction to previous tetanus vaccine
Severe acute illness with high fever (postpone until recovery)
Keep Adrenaline injection ready for rare anaphylactic reactions
Storage
Store at +2°C to +8°C (refrigerated, not frozen)
Protect from light
Keep out of reach of children